Small cell lung cancer sclc accounts for about 10 to 15 of all lung.
Pembrolizumab as first line therapy for metastatic non small cell lung cancer.
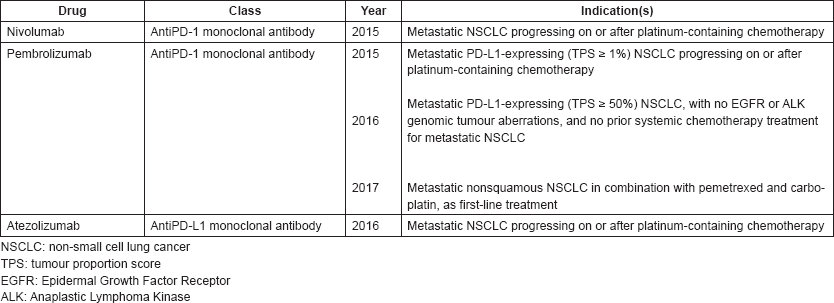
On august 20 2018 fda granted pembrolizumab keytruda regular approval for the initial or first line treatment of patients with metastatic non small cell lung cancer nsclc whose tumors do not have mutations in the egfr or alk genes.
First line therapy for advanced non small cell lung cancer nsclc that lacks targetable mutations is platinum based chemotherapy.
Standard first line therapy for metastatic squamous non small cell lung cancer nsclc is platinum based chemotherapy or pembrolizumab for patients with programmed death ligand 1 pd l1.
Non small cell lung cancer nsclc is the most common type of lung cancer accounting for about 85 of all cases.
Among patients with a tumor proportion score for programmed death ligand 1 pd l1 of 50 or greater pembrolizumab has replaced cytotoxic chemotherapy as the first line treatment of choice.
We investigated overall survival after treatment with pembrolizumab monotherapy in patients with a pd l1 tps of 1 or greater.
The two main types of lung cancer are non small cell and small cell.
A systematic review of recently published studies.
First line pembrolizumab monotherapy improves overall and progression free survival in patients with untreated metastatic non small cell lung cancer with a programmed death ligand 1 pd l1 tumour proportion score tps of 50 or greater.
Real world treatment patterns for patients receiving second line and third line treatment for advanced non small cell lung cancer.
This review describes trials evaluating the monoclonal antibody pembrolizumab an immunotherapy that blocks the interaction between programmed death 1 and programmed death ligand 1 and 2 pd l1 pd l2 as first line therapy for advanced non small cell lung cancer nsclc.
On april 11 2019 the food and drug administration approved pembrolizumab keytruda merck inc for the first line treatment of patients with stage iii non small cell lung cancer nsclc who are.
The change from an accelerated to a regular approval was based on the findings of a large phase 3 trial that included more than 600 patients with.